"PH" redirects here. For other uses, see PH (disambiguation).
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution.[1] Pure water is said to be neutral, with a pH close to 7.0 at 25 °C (77 °F). Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline. pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering and many other applications.In a solution pH approximates but is not equal to p[H], the negative logarithm (base 10) of the molar concentration of dissolved hydronium ions (H3O+); a low pH indicates a high concentration of hydronium ions, while a high pH indicates a low concentration. This negative of the logarithm matches the number of places behind the decimal point, so, for example, 0.1 molar hydrochloric acid should be near pH 1 and 0.0001 molar HCl should be near pH 4 (the base 10 logarithms of 0.1 and 0.0001 being −1, and −4, respectively). Pure (de-ionized) water is neutral, and can be considered either a very weak acid or a very weak base (center of the 0 to 14 pH scale), giving it a pH of 7 (at 25 °C (77 °F)), or 0.0000001 M H+.[2] For an aqueous solution to have a higher pH, a base must be dissolved in it, which binds away many of these rare hydrogen ions. Hydrogen ions in water can be written simply as H+ or as hydronium (H3O+) or higher species (e.g., H9O4+) to account for solvation, but all describe the same entity. Most of the Earth's freshwater bodies surface are slightly acidic due to the abundance and absorption of carbon dioxide;[3] in fact, for millennia in the past, most fresh water bodies have long existed at a slightly acidic pH level.
However, pH is not precisely p[H], but takes into account an activity factor. This represents the tendency of hydrogen ions to interact with other components of the solution, which affects among other things the electrical potential read using a pH meter. As a result, pH can be affected by the ionic strength of a solution – for example, the pH of a 0.05 M potassium hydrogen phthalate solution can vary by as much as 0.5 pH units as a function of added potassium chloride, even though the added salt is neither acidic nor basic.[4]
Hydrogen ion activity coefficients cannot be measured directly by any thermodynamically sound method, so they are based on theoretical calculations. Therefore, the pH scale is defined in practice as traceable to a set of standard solutions whose pH is established by international agreement.[5] Primary pH standard values are determined by the Harned cell, a hydrogen gas electrode, using the Bates–Guggenheim Convention.
pH in its usual meaning is a measure of acidity of (dilute) aqueous solutions only.[1] Recently the concept of "Unified pH scale"[6] has been developed on the basis of the absolute chemical potential of the proton. This concept proposes the "Unified pH" as a measure of acidity that is applicable to any medium: liquids, gases and even solids.
Contents[hide] |
[edit] History
The concept of p[H] was first introduced by Danish chemist Søren Peder Lauritz Sørensen at the Carlsberg Laboratory in 1909[7][8] and revised to the modern pH in 1924 after it became apparent that electromotive force in cells depends on activity rather than concentration of hydrogen ions.[4] In the first papers, the notation had the H as a subscript to the lowercase p, like so: pH.[9]It is unknown what the exact definition of 'p' in pH is. A common definition often used in schools is "percentage". However some references suggest the p stands for “Power”,[10] others refer to the German word “Potenz” (meaning power in German),[11] still others refer to “potential”. Jens Norby published a paper in 2000 arguing that p is a constant and stands for “negative logarithm”;[12] H then stands for Hydrogen. According to the Carlsberg Foundation pH stands for "power of hydrogen".[10] Other suggestions that have surfaced over the years are that the p stands for puissance (also meaning power, based on the fact that the Carlsberg Laboratory was French-speaking), or that pH stands for the Latin terms pondus Hydrogenii or potentia hydrogenii. It is also suggested that Sørensen used the letters p and q (commonly paired letters in mathematics) simply to label the test solution (p) and the reference solution (q).[13]
[edit] Definitions
[edit] Mathematical definition
pH is defined as a negative decimal logarithm of the hydrogen ion activity in a solution.[14]This definition, by itself, is wholly impractical, because the hydrogen ion activity is the product of the concentration and an activity coefficient. To get proper results, the electrode must be calibrated using standard solutions of known activity.
The operational definition of pH is officially defined by International Standard ISO 31-8 as follows:[15] For a solution X, first measure the electromotive force EX of the galvanic cell
- reference electrode|concentrated solution of KCl || solution X|H2|Pt
Measurement of extremely low pH values, such as some very acidic mine waters,[17] requires special procedures. Calibration of the electrode in such cases can be done with standard solutions of concentrated sulfuric acid, whose pH values can be calculated with using Pitzer parameters to calculate activity coefficients.[18]
pH is an example of an acidity function. Hydrogen ion concentrations can be measured in non-aqueous solvents, but this leads, in effect, to a different acidity function, because the standard state for a non-aqueous solvent is different from the standard state for water. Superacids are a class of non-aqueous acids for which the Hammett acidity function, H0, has been developed.
[edit] p[H]
This was the original definition of Sørensen,[10] which was superseded in favour of pH in 1924. However, it is possible to measure the concentration of hydrogen ions directly, if the electrode is calibrated in terms of hydrogen ion concentrations. One way to do this, which has been used extensively, is to titrate a solution of known concentration of a strong acid with a solution of known concentration of strong alkali in the presence of a relatively high concentration of background electrolyte. Since the concentrations of acid and alkali are known, it is easy to calculate the concentration of hydrogen ions so that the measured potential can be correlated with concentrations. The calibration is usually carried out using a Gran plot.[19] The calibration yieds a value for the standard electrode potential, E0, and a slope factor, f, so that the Nernst equation in the formThe difference between p[H] and pH is quite small. It has been stated[20] that pH = p[H] + 0.04. It is common practice to use the term "pH" for both types of measurement.
[edit] pOH
pOH is sometimes used as a measure of the concentration of hydroxide ions, OH−, or alkalinity. pOH is not measured independently, but is derived from pH. The concentration of hydroxide ions in water is related to the concentration of hydrogen ions by- [OH−] = KW /[H+]
- pOH = pKW − pH.
[edit] Applications
Pure (neutral) water has a pH around 7 at 25 °C (77 °F); this value varies with temperature. When an acid is dissolved in water, the pH will be less than 7 (if at 25 °C (77 °F)). When a base, or alkali, is dissolved in water, the pH will be greater than 7 (if at 25 °C (77 °F)). A solution of a strong acid, such as hydrochloric acid, at concentration 1 mol/L has a pH of 0. A solution of a strong alkali, such as sodium hydroxide, at concentration 1 mol/L, has a pH of 14. Thus, measured pH values will lie mostly in the range 0 to 14. Since pH is a logarithmic scale, a difference of one pH unit is equivalent to a tenfold difference in hydrogen ion concentration.Because the glass electrode (and other ion selective electrodes) responds to activity, the electrode should be calibrated in a medium similar to the one being investigated. For instance, if one wishes to measure the pH of a seawater sample, the electrode should be calibrated in a solution resembling seawater in its chemical composition, as detailed below.
An approximate measure of pH may be obtained by using a pH indicator. A pH indicator is a substance that changes color around a particular pH value. It is a weak acid or weak base and the color change occurs around 1 pH unit either side of its acid dissociation constant, or pKa, value. For example, the naturally occurring indicator litmus is red in acidic solutions (pH<7 at 25 °C (77 °F)) and blue in alkaline (pH>7 at 25 °C (77 °F)) solutions. Universal indicator consists of a mixture of indicators such that there is a continuous color change from about pH 2 to pH 10. Universal indicator paper is simple paper that has been impregnated with universal indicator.
| Indicator | Low pH color | Transition pH range | High pH color |
|---|---|---|---|
| Thymol blue (first transition) | Red | 1.2 – 2.8 | Yellow |
| Methyl red | Red | 4.4 – 6.2 | Yellow |
| Bromothymol blue | Yellow | 6.0 – 7.6 | Blue |
| Thymol blue (second transition) | Yellow | 8.0 – 9.6 | Blue |
| Phenolphthalein | Colorless | 8.3 – 10.0 | Fuchsia |
- H2O
 H+ + OH−
H+ + OH−
- CO2 + H2O
 H2CO3
H2CO3  HCO3− + H+
HCO3− + H+
[edit] Examples
Substance | pH |
|---|---|
| Lead-battery acid[21] | < 1 |
| Vinegar[22] | 2.0 |
| Baking soda[22] | 9.0 |
[edit] Calculations of pH
[edit] Strong acids and bases
Strong acids and bases are those that, for practical purposes, completely dissociate (ionize) in water. Hydrochloric acid (HCl) is a good example of a strong acid.A commonly encountered problem is to calculate the pH of a solution of a given concentration of a strong acid. Normally, the concentration of the acid will be very high compared to the baseline concentration of H+ ions in pure water, which is 10−7 molar. Under these conditions, the H+ ion concentration is very nearly that of the acid concentration, and the pH is calculated simply by taking the negative logarithm of that value[23]
For example, for a 0.01M solution of HCl, the H+ concentration can be taken as 0.01M, and the pH is −log10(0.01). That is, pH = 2.
For very weak concentrations, i.e. concentrations around 10−6M or less, the baseline concentration of H+ ions in pure water becomes significant, and must be taken into account.[24] A method of solution is as follows. At equilibrium, any aqueous solution must satisfy the dissociation equilibrium equation for water,
- [H + ][OH − ] = Kw = 10 − 14
- Ca = [HA] + [A − ]
Note that for a given reaction, Ca is constant. This equation is merely saying that the molecules of acid can either be protonated or ionized, but that the total number will stay the same.
For a strong acid which is completely dissociated, [A–] >> [HA], and the [HA] term can be dropped:
- Ca = [A − ]
- [H + ] = [A − ] + [OH − ]
- [H + ]2 − Ca[H + ] − Kw = 0
For example, to find the pH of a solution of 5×10−8M of HCl, first note that this concentration is small compared to the baseline concentration of [H+] in water (10−7). So the quadratic equation derived above should be used.
- pH = 6.89
[edit] Weak acids and bases
The problem in this case would be to determine the pH of a solution of a specific concentration of an acid, when that acid's pKa or Ka (acid dissociation constant) is given.In this case, the acid is not completely dissociated, but the degree of dissociation is given by the equilibrium equation for that acid:
- Ca = [HA] + [A − ]
- [H + ] = [A − ]
- [HA] = Ca − [A − ] = Ca − [H + ]
- [H + ]2 + Ka[H + ] − KaCa = 0
For example, consider a problem of finding the pH of a 0.01M solution of benzoic acid, given that, for this acid, Ka = 6.5×10−5 (pKa = 4.19).
The equilibrium equation for this reaction is
- 0.01M = [HA] + [A − ] = [HA] + [H + ]
- [HA] = 0.01M − [H + ]
- pH = − log[H + ] = 3.11
[edit] pH in nature

Hydrangea macrophylla blossoms are either pink or blue, depending on a pH-dependent mobilization and uptake of soil aluminium into the plants.
[edit] Seawater
The pH of seawater plays an important role in the ocean's carbon cycle, and there is evidence of ongoing ocean acidification caused by carbon dioxide emissions.[26] However, pH measurement is complicated by the chemical properties of seawater, and several distinct pH scales exist in chemical oceanography.[27]As part of its operational definition of the pH scale, the IUPAC defines a series of buffer solutions across a range of pH values (often denoted with NBS or NIST designation). These solutions have a relatively low ionic strength (~0.1) compared to that of seawater (~0.7), and, as a consequence, are not recommended for use in characterising the pH of seawater, since the ionic strength differences cause changes in electrode potential. To resolve this problem, an alternative series of buffers based on artificial seawater was developed.[28] This new series resolves the problem of ionic strength differences between samples and the buffers, and the new pH scale is referred to as the total scale, often denoted as pHT.
The total scale was defined using a medium containing sulfate ions. These ions experience protonation, H+ + SO42− ⇌ HSO4−, such that the total scale includes the effect of both protons (free hydrogen ions) and hydrogen sulfate ions:
- [H+]T = [H+]F + [HSO4−]
- [H+]F = [H+]T − [HSO4−] = [H+]T ( 1 + [SO42−] / KS* )−1
Another scale, known as the seawater scale, often denoted pHSWS, takes account of a further protonation relationship between hydrogen ions and fluoride ions, H+ + F− ⇌ HF. Resulting in the following expression for [H+]SWS:
- [H+]SWS = [H+]F + [HSO4−] + [HF]
The following three equations summarise the three scales of pH:
- pHF = − log [H+]F
- pHT = − log ( [H+]F + [HSO4−] ) = − log [H+]T
- pHSWS = − log ( [H+]F + [HSO4−] + [HF] ) = − log [H+]SWS
[edit] Living systems
| Compartment | pH |
|---|---|
| Gastric acid | 1 |
| Lysosomes | 4.5 |
| Granules of chromaffin cells | 5.5 |
| Human skin | 5.5 |
| Urine | 6.0 |
| Neutral H2O at 37 °C | 6.81 |
| Cytosol | 7.2 |
| Cerebrospinal fluid (CSF) | 7.3 |
| Blood | 7.34–7.45 |
| Mitochondrial matrix | 7.5 |
| Pancreas secretions | 8.1 |
The pH of blood is usually slightly basic with a value of pH 7.365. This value is often referred to as physiological pH in biology and medicine.
Plaque can create a local acidic environment that can result in tooth decay by demineralisation.
Enzymes and other proteins have an optimum pH range and can become inactivated or denatured outside this range.
The most common disorder in acid-base homeostasis is acidosis, which means an acid overload in the body, generally defined by pH falling below 7.35.[citation needed]
In the blood, pH can be estimated from known base excess (be) and bicarbonate concentration (HCO3-) by the following equation:[33]
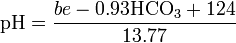
[edit] Extremes of pH
pH is normally measured in a range of 0-14. However, due to the way pH is calculated, it's possible to have negative pH and pH above 14.[34] However, it should be noted that reported values outside the range 0-14 are somewhat controversial; as measuring pH beyond this range becomes difficult.Runoff from mines/mine tailings can produce some of the most acidic pHs ever reported; with negative pHs measured and reported in the literature as low as pH -3.6.[35]


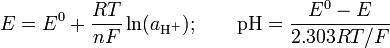

![E = E^0 + f\frac{RT}{nF} \ln[\mbox{H}^+]](http://upload.wikimedia.org/math/6/5/4/6547a53032ebdffc5797c8701894cd06.png)

![[H^+] = C_a + \frac{K_w}{[H^+]}](http://upload.wikimedia.org/math/6/0/e/60e44409aebe2acc518341cd7cdbb923.png)
![[H^+]^2 - 5 \times 10^{-8} [H^+] - 10^{-14} = 0](http://upload.wikimedia.org/math/4/c/2/4c2d5b9df79a1c6b72a68d8af3853f3b.png)
![[H^+] = 1.28 \times 10^{-7}](http://upload.wikimedia.org/math/9/1/e/91e9c640b43ee428a1e056cdee7b4411.png)
![K_a = \frac{[H^+][A^-]}{[HA]}](http://upload.wikimedia.org/math/1/7/9/1797ef4d5bb0d98ca3803b0932e09832.png)
![K_a = \frac{[H^+]^2}{C_a - [H^+]}](http://upload.wikimedia.org/math/2/b/d/2bde22b9d62477afbdd8884e1c1e0c40.png)
![6.5 \times 10^{-5} = \frac{[H^+][A^-]}{[HA]}](http://upload.wikimedia.org/math/f/d/6/fd6dc2a90fe106d36cb132de10aeae50.png)
![6.5 \times 10^{-5} = \frac{[H^+]^2}{[HA]}](http://upload.wikimedia.org/math/c/2/7/c27a5831da9281c3b6e9fe1e4e6f9bbe.png)
![6.5 \times 10^{-5} = \frac{[H^+]^2}{0.01 - [H^+]}](http://upload.wikimedia.org/math/d/2/7/d275240bc13f75f96857633d2d8526b7.png)
![[H^+]^2 + 6.5 \times 10^{-5}[H^+] - 6.5 \times 10^{-5} \times 0.01 = 0](http://upload.wikimedia.org/math/e/4/d/e4dcbd72f271359936d604b91600cec7.png)
![[H^+] = 7.74 \times 10^{-4}](http://upload.wikimedia.org/math/d/9/1/d9162f8b93e1cfa131c07597e1033169.png)


Đăng nhận xét